By Hannah Foote and Madison Atkinson
LOS ANGELES – Scientists in Flagstaff are working to develop a test that doctors and hospitals could use to diagnose coronavirus, which could help medical professionals respond to the global outbreak more quickly.
For now, the only test available in the U.S. was developed by the Centers for Disease Control and Prevention, and medical professionals send samples to approved labs to determine whether a patient’s illness is coronavirus.
Researchers at the Translational Genomics Research Institute, known as TGen, are hoping to create a test that could be more broadly implemented, by making it available to hospitals and doctors to conduct their own testing.
“We want to make sure that we’re ready to be able to respond,” said Dr. David Engelthaler, director of the northern division of TGen.
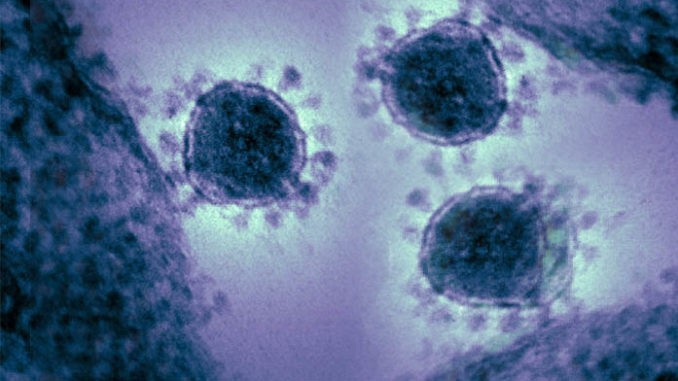
TGen North is using genetic information to map the RNA of the respiratory virus to determine whether the patient has a strain, what strain they have, and whether there is a mutation present, Engelthaler said. The novel coronavirus, identified as 2019-nCoV, was first detected in China in late December.
To date, only the CDC’s test is being used, and the Food and Drug Administration issued an emergency use authorization Feb. 4 to allow more CDC-qualified labs to test samples.
“This continues to be an evolving situation, and the ability to distribute this diagnostic test to qualified labs is a critical step forward in protecting the public health,” FDA Commissioner Stephen M. Hahn said in a press release.
With 40,554 confirmed cases globally, there are 13 novel coronavirus cases in the United States, as of Monday.
TGen researchers have previously assisted in developing tests for other strains of coronavirus, including SARS and MERS.
TGen’s test is in the approval process at the Food and Drug Administration, which typically takes months to complete – however, the FDA has a shorter approval process for public health emergencies. For use in other countries, however, the process of approval is expected to take longer.
“If we’re going to work with partners in other parts of the world, we’re going to have to work with their own regulatory agencies,” Engelthaler said.
TGen isn’t the only company racing to develop a test: Biotech company Novacyt is also seeking emergency use approval from the FDA for its test and researchers in Hong Kong claim they have a test that gives a diagnosis in 40 minutes. Public Health England also launched a test Monday for 12 laboratories in the United Kingdom.
Video by Madison Atkinson/Cronkite News
[metaslider id=65385]
